THE CLAIM HAS BEEN made that whereas acceptable evidence of microevolution exists, there is no acceptable evidence for macroevolution The microevolutionary changes conceded are changes in gene frequencies or genetically based adaptations, which can be demonstrated in short-term scientific studies. These include changes in the frequency of dark morphs in moths, and changes in the age of first reproduction in fish as the result of the selective actions of predators on fish.
Macroevolution, however, is seen as unsubstantiated by critics of evolutionary theory. It is not seen how a process of macroevolution could produce new higher categories of life such as bird, butterflies, and flowering plants, as well as any unique and well-developed structures they possess such as brains, wings, and flowers.
Macroevolution suffers, in this view, from unconvincing evidence, missing evidence, and counter-evidence. Deemed unconvincing is the evolutionary biologists' claim that the processes that led to observable short-term changes in the genetic complements of species (and the traits governed by these genes) also led over millions of years to bigger changes, greatly modified structures with new uses, and new kinds of organisms. Also deemed unconvincing is the occasional fossil intermediate-the odd whale with legs here and the reptile with feathers there.
The missing evidence, in this view, is explained away as gaps in the fossil record. The missing "proof' would have to be a chain from ancestor to very different descendent of adapted intermediates, not overlapping in time, each superior to its predecessor.
The counter-evidence for macroevolution is regarded to be the overlapping in time of presumed ancestral and descendent species. Other counter-evidence is held to be the apparent sudden appearance- suggesting creation-of new forms, and of life itself.
Given, finally, that the evidence for macroevolution is so bad, the reason that so many scientists stand behind it must be political. There is a struggle for cultural domination: Science or God, Evolution or Creation Scientists must exclude an actively creating or otherwise involved God because, if they didn't, it would mean the death of science. To win, scientists push the dogma of metaphysical naturalism, which states that knowledge can come only through the methods of investigation of natural science.
An example of this is an evolutionary system set up by Professor Thomas Ray of the University of Delaware on a computer (Lewin 1992). It allows him to run the model process more than once, right from the beginning. Professor Ray programmed a digital organism that was represented by a line segment of a given length and color on the monitor screen, with a definite head and tail and a specific "genetic" code for self-replication in a sequence in between. If it could find space in the form of a physical location in computer memory, and if it could obtain an analog of sufficient energy in the form of time on the computer, it would be able to carry out the replication programmed by its code. Indeed, in a short amount of time, measured in computer generations, the screen was filled with copies of the segment.
The system was also such that random, that is, unpredictable, enters in replication would occasionally occur. These replication errors had no preordained adaptive value: they were simply random changes. After a while, segments of other lengths and slightly different sequences began to appear. This was minor novelty, so far. Then Professor Ray pressed the button to run the program overnight, and went home to bed.
What did he find the next morning? He found a high degree of diversity in his community of digital organisms. He found adaptive diversity in the form of ingenious ways of getting replicated, given the limited space and computer time and the nature of the competing segments. He found novelty, things he had not explicitly programmed that initial segment to do. There were segments that alone were unable to replicate, but in coordinated groups were able to do so (mutualistic organisms). There were organisms that consumed the code of other organisms (predators), thereby gaining time, the all-important currency in Ray's universe. There were even small segments, that through mutation had lost their own ability to replicate (they no longer had a replication code of their own), but that nevertheless persisted. They persisted inside the sequence of larger "host" digital organisms and used the host's mechanism of replication to accomplish their own (parasitic organisms). There were other small segments that actually modified the replicating of the host to serve their own parasitic needs better (viruslike organisms). There was a host that through chance errors developed a way of resisting invasion by segments that had once parasitized their forerunners. This new subtype increased at the expense of the forerunners, and came to predominate, at which time the parasites disappeared altogether, not having stumbled on a way to overcome the defense. Thus the so-called "arms race," so common in macroevolution, was underway and was observable. Overall diversity waxed, sometimes in surges of new production, and waned, sometimes in crashes of diversity.
Certainly, this is not the same as populating the planet earth with organic life. But this computer exercise succeeded in demonstrating that the evolution of complex levels of diversity is possible using only the most basic ingredients of the recipe for evolution. A simple mechanism of inheritance and replication, and a mechanism to generate variety through replication errors-when coupled with selection-were sufficient to produce a quite remarkable menagerie of digital organisms occupying very different ecological roles in the digital community. These basic ingredients were all that was needed to produce adaptive diversity quite simply and easily, as well as levels of complexity not present or anticipated at the outset.
What is nice about Professor Ray's model of evolution is that it lets us explore the question of how unique the diversity and ecological complexity of life on earth is. We have only one history of life to examine (a sample size of one). Ray's exercise is the first generation of computer experiments to understand the degree to which the patterns in evolution that occurred on earth might be more general and likely to have occurred elsewhere in the universe. Such models can also be used to test how prior evolutionary developments promote or retard the subsequent development of diversity, innovation, and complexity in an assemblage of species. In contrast, the history of life on earth is not amenable to experimentation.
So, what is there to see in the unique history of life on our planet, if we could watch the whole parade? Chains of temporally adjacent ancestors and descendants all progressively improving? Not exactly. We would see overlapping of ancestral and descendent species. Current understanding is that speciation involves the splitting of a species into two species, mother and daughter. The process is complete when individuals from the two species are no longer able to interbreed. Mother and daughter species can be contemporaries just as mothers and daughters can be. We can today find species pairs that are the two prongs of a split, like Traill's flycatcher and its sister species.
We would also not be able to show that each succeeding form was always or obviously superior to the last. This implies progression. The evidence suggests instead that quite a bit of evolutionary change is neutral, and that the latest form should not be viewed as the necessarily best in any absolute sense. Those life forms that persist into the next interval are a combination of luckier and "better than the competition" at surviving and reproducing in the given environment.
Paleontologists estimate that as many as 99.9% of all species that ever lived are extinct (Raup 1991). Estimates of the number of species alive today range up to 30 million, mostly insects (Erwin 1988), but more conservative estimates range from 2.5 to 5 million species. Suppose for sake of discussion we take the most conservative estimate, and state that approximately 2.5 billion total species existed over the history of the Phanerozoic.
The next point to consider is the probability of discovering or "sampling" a given species as a fossil. Many factors affect this likelihood. Organisms with hard parts are more likely to fossilize than soft-bodied ones. Creatures that inhabit aquatic or marine environments are more likely to fossilize than organisms found in dry, upland habitats. Large-bodied organisms are more likely to fossilize than small ones. Abundant or widespread species are more likely to be sampled than rare or localized species.
While these factors are important, probably the most important of all is the geological lifespan of the species. Species differ hugely in lifespans. A species that is around for tens of millions of years is much more likely to be sampled than a species lasting but a few thousand, regardless of its body size, abundance, or geographic range.
Therefore, we need to consider the distribution of lifespans of species in the fossil record. Nearly two decades ago, Van Valen (1973) constructed survivorship curves for extinct species in a number of vertebrate groups. He discovered that, to a first approximation, these curves were exponential. This implied that extinction was a stationary Poisson process, and that the probability of extinction was approximately constant per unit time. From this and later work, the average lifespan of a species was estimated to be approximately 4 million years. Note, however, that this estimate is derived from those species that were sampled by the fossil record, which is likely to be an overestimate, biased in favor of the longest surviving species to begin with. How can we correct for this bias?
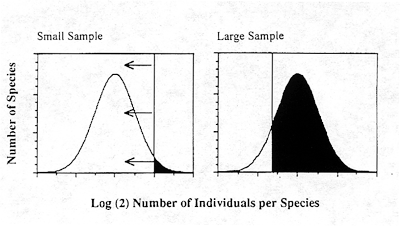
The answer to this question lies in discoveries made in the subjects of community ecology and biogeography. Whenever ecologists have counted the numbers of individuals in species in ecological communities, they have found that they are best described by a lognormal distribution (Preston 1948, 1962) (Figure 1). When species frequencies are counted in doubling abundance classes (number of individuals per species in octaves), and when the sample size is large enough, a bell-shaped curve of species numbers by abundance class is observed (e.g., Hubbell and Foster 1983). Biogeographers have discovered that the ranges of plant and animal species are similarly lognormally distributed (Brown and Gibson 1983). The lognormal arises in natural autocatalytic systems such as reproducing and dispersing populations, in which many normal random factors act multiplicatively on growth. For similar ecological and biogeographical reasons, the lifespans of species are expected to be lognormally distributed as well. It is an established principle that small, local populations are more extinction-prone than large, widespread species. If we assume that risk is inversely proportional to geographic range, then lifespans would be expected to follow the lognormal. Because of the strong impact of Van Valen's (1973) work, paleontologists have largely focused on the exponential distribution as a model of lifespan and risk of extinction, and have ignored the lognormal. However, the exponential distribution is a fair approximation to the righthand tail of the lognormal distribution. Preston (1948) noted that a nearly exponential distribution will be seen in small sample sizes of a lognormal because only the most abundant species will be sampled. Only as sample sizes increase will the mode of the lognormal be revealed. He called this effect of sample size an "unveiling" of the lognormal. It is as if a "veil line" moved from right to left across the lognormal, revealing more and more of the lognormal, ultimately unveiling the mode of the distribution (Figure 1).

Figure 1. Lognormal distribution of the relative abundance of species. When sample sizes are small (left panel), only the very abundant species are captured (rightmost tail of lognormal), to the right of the vertical line, called the "veil line" by Preston. As the sample size is increased the veil line moves to the left, as shown in the right panel. as increasingly rarer species are added to the sample. When sample sizes are small, the visible portion of the lognormal appears approximately exponential (the righthand tail). The area of species ranges is also lognormal. Population lifespans increase with population size and geographic range, so lifespans are also expected to be lognormal.
The lognormal result for the fossil record is shown in Figure 2, with the placement of the veil line far toward the extreme of the righthand tail. This graph immediately reveals what a tiny sample of all 2.5 billion
Phanerozoic species the fossil record contains On average only species with lifespans greater than 1 to 2 million years (221 years) have been sampled. At the present, some 250,000 extinct species have been found in the fossil record. Suppose for sake of argument that paleontologists are extremely fortunate, and ultimately increase the number of known fossil species to 350,000. Even assuming such good fortune, this is a discovery rate of just 1 fossil species per 7,000 species that ever lived.
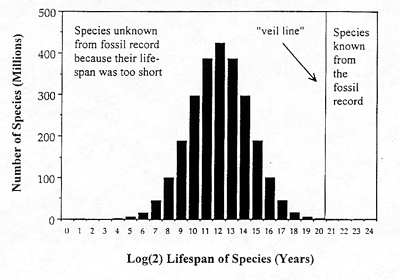
Figure 2. The lognormal applied to the data from the Phanerozoic era. Of the estimated 2.5 billion species that ever lived during this period only extremely long-lived species were sampled by the fossil record. Because of the scaling it is difficult to see that there is still a portion of the curve to the right of the veil line. The total number of species under the curve is 2.5 billion. The number of species known from the fossil record, or ultimately knowable, is perhaps on the order of 350,000.
We can display at an expanded vertical scale just the righthand tail of the lognormal from Figure 2, to show the fact that the apparent lifespan of species in the fossil record is approximately exponential (Figure 3). When we compare the observed average lifespan of fossil species (about 4 million years) with that calculated from the righthand tail of the lognormal. we have an independent check on the time scaling of the lognormal. This gives an expected lifespan of 3.7 million years (Figure 3).
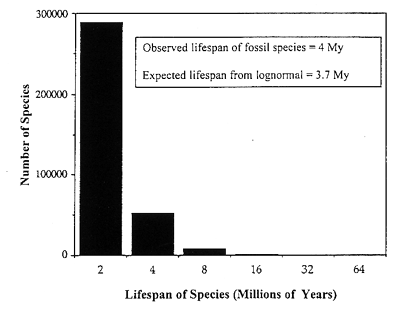
Figure 3. Righthand tail of the lognormal in Figure 2, to the right of the veil line, with an expanded vertical axis, showing the number of fossil species with given Lifespans (doubling classes in millions of years) The observed and expected lifespans (4.0 and 3.7 million years) for known fossils agree reasonably well.
Although the mean lifespans are well matched, we need a more rigorous comparison of how well the expected distribution of lifespans corresponds with the data on actual lifespans of fossil species. A considerable amount of research has been done on the question of lifespans in the fossil record since the publication of Van Valen's original paper in 1973. Perhaps the best data set available is that of Professor Sepkoski of the University of Chicago, on the survivorship of 17,505 genera, as reported in Raup (1991). It would be handier to have data on individual species, but the generic data will serve our present purposes. These improved data reveal that Van Valen's assertion of exponential lifespan distributions is, in fact, not precisely correct (figure 4).
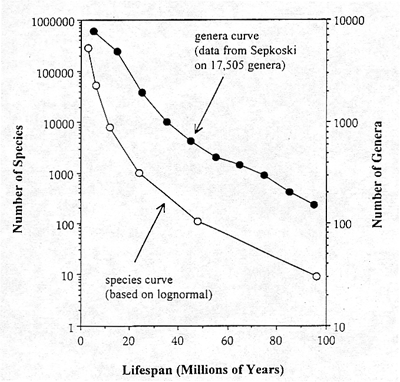
Figure 4. Comparison of the distribution of lifespan observed in 17,505 fossil genera with that expected for individual species from the lognorrnal The genera curve is flatter than the species curve, as expected, since genera are expected to survive longer. Note that both curves exhibit the curvilinearity predicted from the lognormal, but not from the exponential distribution.
The actual curves of log number of surviving genera begin to flatten out when really long-lived genera are considered. This is the shape that is expected from an underlying lognormal distribution, not from an exponential distribution. The species curve predicted from the lognormal distribution is also shown in Figure 4. It shows that lifespans for individual species are shorter than for genera, as would be expected, but it also displays the curvature of the lognormal tail, not the straight line that would be expected for an exponential survivorship curve.
The main conclusion of this analysis is that fossil species are only a trivial fraction of all species that have ever lived, and therefore it is only with the greatest luck that we should find missing links, let alone a nearly continuous sequence of ancestral and descendent forms.
All this points up that the vast majority of extinct species will never be found, because they rarely met the conditions for fossilization, or because their presence was ephemeral, or because they were too rare or local in distribution. There is reason to believe, moreover, that "missing links" will be under-represented among available fossil puzzle pieces. According to current theory, the bursts of adaptive radiation that punctuate the periods of evolutionary stasis tend to involve species with more rapid generation times. Such species in turn tend to be smaller species with more delicate structures that are less likely to be preserved, and small populations at the periphery of the geographic range of the group, whose individuals are less likely to be fossilized because of their rarity.
What the paucity of links and unequivocal ancestors does not do is falsify the theory of evolution. Rather, the fossil record, for all its shortcomings, is highly supportive. What is telling is what we don't see: Devonian sharks with feathers and wishbones, mammals in strata with the first land plants, intermediates between trilobites and titanotheres. Instead, each new discovery corroborates the picture of the history of life in its broad outlines, a picture that makes its greatest sense in the context of adaptive evolution. A fossil whale with legs, by itself is inadequate evidence for construction of a theory of descent by evolution. A whale with little bat wings would certainly merit concerted study, but by itself would hardly be fatal. Hundreds or thousands of such anomalies would be a serious problem, but in fact, we don't get these. What we get are new finds like the Chinese fossil bird (Sereno and Chenggang 1992), which is nicely intermediate in time and structure between Archaeopteryx and more modern birds. The striking thing is that all serendipitous finds fit in; the likelihood of this happening without macroevolution based on microevolutionary processes is vanishingly small.
A second thing lends a theory validity in the eyes of scientists. A theory gains validity to the extent it generates successful predictions in new areas. that is, in areas beyond the phenomena the theory was originally developed to explain. Another way to put it is that healthy theories pass tests coming from new directions. Validity of this kind accrues to evolutionary theory. Advances in molecular genetics, for example, have made it possible to "read" the genetic sequences of organisms. The patterns found confirm predictions of evolutionary theory.
A third feature that distinguishes theories with broad acceptance is that they point the way to profitable new avenues of inquiry. Social behavior, for example, was a long neglected field of biology. Evolutionary biologists, of course, recognized that in many circumstances individuals with genes for effective forms of parental care would have greater success in leaving descendents than individuals without such genes, meaning, therefore, that parental care could be a naturally selected trait. A breakthrough came, however, when biologist William Hamilton (1964) realized that an individual shares genes in common by descent not only with its offspring, but with all other kin as well, with more genes held in common the closer the degree of kinship. He then developed a body of ideas expressing the circumstances under which individuals would be expected to sacrifice, even to the point of ending their lives and all future chance at reproduction, for the sake of kin. William Hamilton's evolutionary thinking turned out to be seminal; it provoked the greatest burst of productive study of the social behavior of animals the world had ever seen. indeed, kin relationships may be the single most crucial factor that structures animal societies. With evolutionary theory pointing the way, species after species was found to have the ability to recognize kin and to use this ability. Mice for example, treat experimentally introduced strangers that happen to be kin differently than they do experimentally introduced strange mice that are non-kin. They can tell kin by their smell.
I will give one last example of the productive, predictive, and unifying power of
evolutionary theory. The Galápagos islands are remote oceanic islands, never attached to
any mainland. They were made, one by one, of cooled lava that welled up as a tectonic
plate passed over a "hotspot," or weak place in the earth's mantle. Each island was barren
at first, but was eventually colonized by terrestrial species from the mainland or from
neighboring islands. Descendents of the colonists then evolved in situ.
Instead of jettisoning evolutionary theory, the investigators took a new tack. They suggested that older islands in the Galapagos chain may have existed, but became submerged. Sure enough, Christie et al. (1992) report finding drowned islands downstream from the Galipagos hotspot. These islands extend the time for speciation another 2-6 million years. Moreover, on the basis of geophysical evidence, the geologists predict that older islands yet will be found. They consider it likely that Galapagos islands have existed during the entire 80-90 million year history of the hotspot.
This example shows all the kinds of strengths exhibited by the theory of evolution: consistency with information in several fields (immunology, biogeography, and geophysics), predictive power, and independent corroboration. To scientists, the theory of evolution is convincing on its own merits. Nothing else needs to be invoked, including desperation or adherence to dogma.
Scientists are not disciples of metaphysical naturalism, which holds that science is the only way of knowing. All scientists have other ways of knowing if only because they are people with human thoughts and concerns. In addition, scientists span the range of religiousness, from the truly devout for whom the everyday world is suffused with spiritual light, to active atheists.
Phillip Johnson in the 1990 booklet Evolution as Dogma (Dallas: Haughton), is concerned by the notion that science is absolute truth, which might imply that other forms of knowledge are fantasy. This is a misreading. Science tells us what the natural world appears to be like, based upon our senses and the instruments we devise to extend our senses, as probed using scientific methods in our tiny comer of spacetime. The scientific method leads us to ask questions and to test hypotheses, and few would question its practical and aesthetic contribution to human welfare. Anyone who says that scientific knowledge equals Absolute Truth, however, is confusing the map with the territory.
Those concerned that science leaves no room for God are similarly misled. On a number of profound issues and concerns, science is of no use at all. On the compelling question of how to be a good human being, science is silent. On the question of the meaning of existence, it is likewise mute. Nonscientific ways of knowing are crucial to our well being. Nevertheless, I myself am flattered (a human response) to think that we humans are capable of creating for ourselves a complex reality that includes science, and I would hope that the complex reality of other people can also include science. Then, our moral sense-separate from science can guide our use of scientific knowledge for the welfare of humankind and our planetary home.
Faced with the enormity of the present extinction crisis, the question of whether species were specially created or evolved seems almost quaint. What better goal to bring the communities of creationists and evolutionary biologists together than to commit to saving the wonderful diversity of life on earth? Creationists should be among the most ardent conservationists of them all.
Christie. D. M., R. A. Duncan, A. R. McBirney, M. A. Richards, W. M. White, K. S. Harpp and C. G. Fox 1992. Drowned islands downstream from the Galipagos hotspot imply extended speciation times. Nature 355:246-248.
Erwin, T. L. 1988. The tropical forest canopy. In: E. O. Wilson (ed.) Biodiversity. National Academy Press. Washington, D.C.
Hamilton, W. D. 1964. The genetical evolution of social behaviour. J. Theor. Biology 7:1 -32.
Hubbell, S. P. and R. B. Foster 1983. Diversity of canopy trees in a neotropical forest and implications for conservation. In: S. L. Sutton, T. C. Whitmore and A. C. Chadwick (eds.) Tropical Rain Forest: Ecology and Management. Blackwell, Oxford.
Johnson, P. 1990. Evolution as Dogma: The Establishment of Naturalism, Haughton Publishing Co., Dallas.
Lewin, R. 1992. Life and death in a digital world. New Scientist: 36-39.
Preston, F. W. 1948. The commonness, and rarity, of species. Ecology: 29:254-283.
Preston, F. W. 1962. The canonical distribution of commonness and rarity. Ecology 43:185-215.
Raup, D. M. 1991. Extinction: Bad Genes or Bad Luck? W. W. Norton, New York.
Sereno, P. C. and R. Chenggang 1992. Early evolution of avian flight and perching: New evidence from the Lower Cretaceous of China. Science 255:845-848.
Van Valen, L. 1973. A new evolutionary law. Evolutionary Theory 1:130.
Wyles, J. S. and V. M. Sarich 1983. Are the Galapagos iguanas older than the Galápagos? Molecular evolution and colonization models for the archipelago. In: Galápagos Organisms. Pacific Division of the AAAS, San Francisco.